Example For Zeroth Law Of Thermodynamics
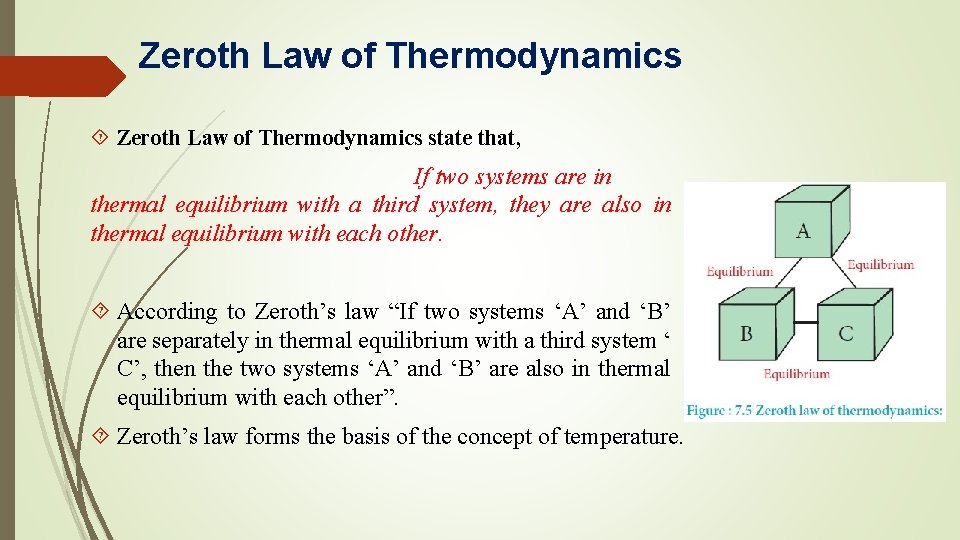
Two systems are in thermal equilibrium if and only if they have the same temperature. When body A is in equilibrium with body B and also separately in equilibrium with body C then B and C will also be in thermal equilibrium with one another.

An Engineering Refresher The Laws Of Thermodynamics Machine Design
If my cup of coffee has the same tem.
Example for zeroth law of thermodynamics. Formally the Zeroth Law of Thermodynamics can be stated as. And the most common example is Thermometer. 1 Similarly if system B is in thermal equilibrium with system C then.
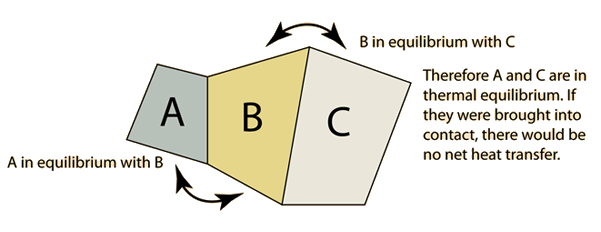
The double arrow represents thermal equilibrium between systems. Accordingly thermal equilibrium between systems is a transitive relation. According to David McKee a physics professor at Missouri Southern State University the Zeroth Law tells us that no matter how much energy two systems have.
If A and C are in thermal equilibrium with B then A is in thermal equilibrium with C. Quantum mechanics in the limit 0but. The Zeroth Law says that the temperature defines the direction of heat flow Examples of Applications of the Zero Law of Thermodynamics Ice and Water.
Zeroth Law of Thermodynamics. Consider that there are three bodies A B and C. Two systems are said to be in thermal equilibrium with respect to each other if they are linked by a wall permeable only to heat and they do not change.
The zeroth law of thermodynamics can be illustrated with a simple example. The Zeroth Law of Thermodynamics is probably the most intuitive and easy to understand of all the laws of thermodynamics. Temperature is the quantity that is always the same for all systems in thermal equilibrium with one another.
Then zeroth law establishes that systems B and C will also be in thermal equilibrium. Consider three systems A B and C. According to Zeroth law of thermodynamics if system A is in thermal equilibrium with a system C then.
For example when a thermometer is kept in contact with a human body suffering from fever the mercury gains heat and it expansion in the thermometer tube. It is a familiar fact that classical mechanics is an implication of quantum mechanicsis quantum mechanics in the limit that the quantum numbers are large formally. This explanation clearly defines the zeroth law of thermodynamics.
When we have a fever we visit the doctor and the doctor uses to check our body temperature with a thermometer. Separately systems A and B are in thermal equilibrium. 0th law of thermodynamics helps us in finding the temperature of a human body.
Zeroth Law Of Thermodynamics Examples Application. It states that if two systems A and B are in equilibrium with a third system C then A and B are in equilibrium with each other. For example the zeroth law of thermodynamics states that if two bodies are in thermal equilibrium with a third body they are also in thermal equilibrium with each other.
Zeroth Law of Thermodynamics Zeroth law of thermodynamics. The temperature of system A temperature of system C. Zeroth law of thermodynamics.
The same goes for Examples Of Zeroth Law Of Thermodynamics. Zeroth Law of Thermodynamics. Fundamental notions of classical thermodynamics and the ZEROTH FIRST SECOND LAWS Introduction.
The temperature of system B temperature of system C. 1When a body A is in thermal equilibrium with another body b and also separately in thermal equilibrium with a body C then body B and C will. I mean there is so many zeroth law of thermodynamics examples that can be seen in our everyday life.
Let the systems A and C be in thermal equilibrium. After few seconds mercury achieves thermal equilibrium with the human body. The zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third then all three are in thermal equilibrium with each other.
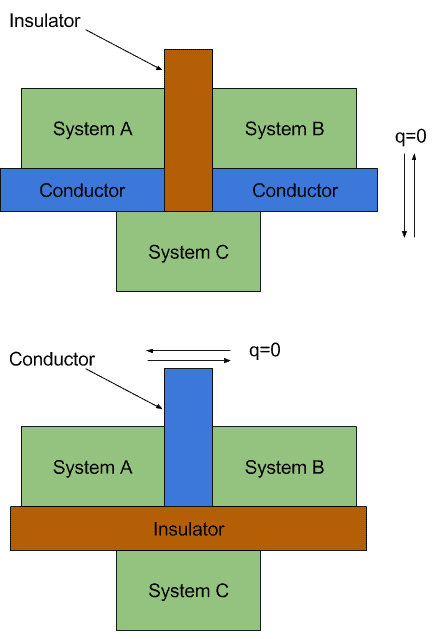
The zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third system then they are in thermal equilibrium with each other. If object A and B are separately in thermal equilibrium with a third object C then A and B are in thermal equilibrium with each other. By this stage of physics we will have spent a lot of time learning about properties of materials conductors have resistance fluids are v.
Celsius temperature scale 0 C is set to be the freezing point of water 100 C is set to be the boiling. Zeroth Law of Thermodynamics Basics. If systems A and C are in equilibrium and systems A and B are in equilibrium then systems B and C are in equilibrium.

Chapter 19 Temperature 19 1 Temperature And The Zeroth Law Of Thermodynamics 19 2 Thermometers And The Celsius Temperature Scale 19 3 The Constant Volume Ppt Download

Zeroth Law Of Thermodynamics W3spoint
What Is Zeroth Law Of Thermodynamics Definition

The Laws Of Thermodynamics The Zeroth Law If Object 1 Is In Thermal Equilibrium With Object 2 And Object 2 Is In Thermal Equilibrium With Object 3 Ppt Download
What Is Zeroth Law Of Thermodynamics And What Are Some Examples Quora
Law Of Thermodynamics Juwel Rana Lecturer Dept Of
Http Courseware Cutm Ac In Wp Content Uploads 2020 06 Thermodynamic Primciple Pdf

Example And Uses Of Zeroth Law Of Thermodynamics

Examples Of Zeroth Law Of Thermodynamics Real Life Examples
An Introduction To Thermodynamics Laws
Zeroth Law Of Thermodynamics Definition History And Examples Physics In My View

The Zeroth Law Of Thermodynamics Video Lesson Transcript Study Com

What Is Zeroth Law Of Thermodynamics 5 Best Examples

Thermal Equilibrium Energy Education
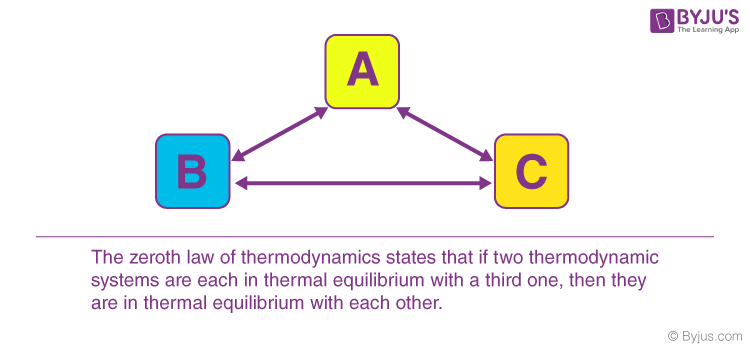
Zeroth Law Of Thermodynamics Examples Application Thermal Equilibrium
What Is The Zeroth Law Of Thermodynamics Quora
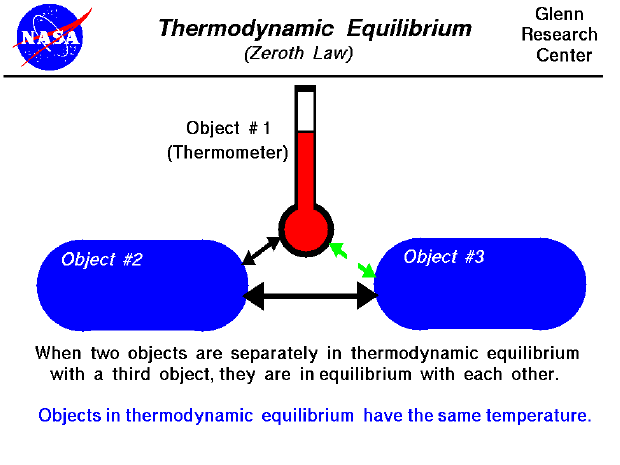

Post a Comment for "Example For Zeroth Law Of Thermodynamics"